Which Statement Best Describes the Quantum Model of the Atom
It makes predictions based on Schrodingers wave equation. B It is a region in space that has a precise shape and.
The Quantum Mechanical Model Of The Atom Article Khan Academy
The best we can know are probabilities.
. Quantum Mechanical Model of Atom. An atom consists of a central nucleus with proton neutrons and electrons orbiting in levels of high probability. An electron transition from a lower energy level to a higher energy level results in an emission of a photon of light.
According to the new current Quantum atomic model An orbital is a region of space in an atom where the. Which statement describes ONLY the Quantum Mechanical model of the atom. In Bohrs model of the atom where are the electrons and protons located.
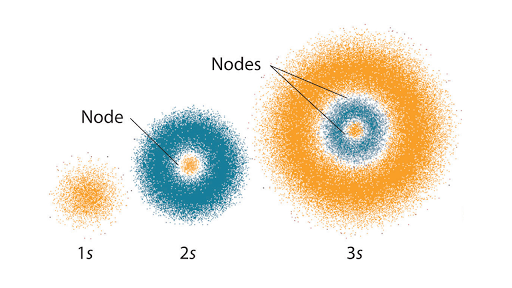
When an atom emits light electrons fall from a higher orbit into a lower orbit. The solution of the wave equation brings the idea of shells sub-shells and orbitals. N indicates the energy level of an electron in the atom.
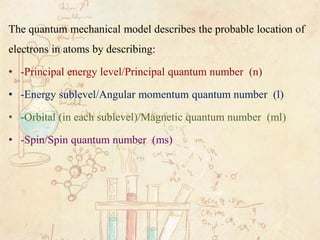
The energy of an electron in an atom is quantized ie. This question requires the examinee to demonstrate knowledge of the characteristics and arrangement of subatomic particles and historical and contemporary models of the atom. The principal quantum number n specifies each unique energy level.
It describes an electron probability distribution that determines the most likely location of an electron. Which statement best describes the quantum mechanical modern model of the atom. Start studying Chapter 8.
I II and III. It is the currently accepted atomic model. B It is a region in space that has a precise shape and is completely filled hva dense electron cloud.
Which statement describes only the quantum mechanical model of the atom. Quantum mechanics is based on Schrödingers wave equation and its solution. Select all that apply.
An electron orbits in spherical orbits at discrete distances from the nucleus. A It is a spherical or dumbbell-shaped route traced by the electron in its rapid movement. Bohrs atomic model is also commonly known as the ____ model.
The electrons and protons move throughout the atom. Due to this high probability orbits are replaced by the term orbital. A Energy Is quantized B Has discrete energy levels.
Which statement correctly describes the quantum number n with reference to the Bohr model of the hydrogen atom. The majority of the mass of an atom is outside of the nucleus. The electrons and protons move throughout the atom.
Which statement below does NOT follow the Bohr Model. Which of the following types of spectra have dark lines. The Quantum-Mechanical Model of the Atom.
Atomic Spectra and Quantum Theory. All of the following statements are true about Bohrs model of the atom except which one of the following. None of the above D.
Which of the following describes the quantum-mechanical model of the atom. The quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded by an electron. The electrons occupy fixed positions around the protons which are at the center of the atom.
Which statement best describes an occupied orbital according to the quantum mechanical model. Electrons exist in specific quantized orbits. The quantum mechanical model describes a probability cloud for the electrons position with respect to the nucleus the shape of the cloud any special orientations and the spin of.
Start studying Chapter 8. Although the Bohr model of the atom accounted for the stability of atoms and the quantization of energy in discrete packets the quantum mechanical model of the atom introduced the idea. The Bohr model successfully predicted the energies for the hydrogen atom but had significant failures that were corrected by solving the.
The energy emitted from a relaxing electron. Due to this high probability orbits are replaced by the term orbital. According to the Bohrs atomic model the electrons revolve around the nucleus in a fixed orbit.
Has sub levels within the energy levels D Sometimes referred to as the planetary. Only specific energy values are possible. Thinking about electrons as probabilistic matter waves using the de Broglie wavelength the Schrödinger equation and the Heisenberg uncertainty principle.
A energy is quantized. The quantum mechanical model of the atom Introduction to the quantum mechanical model of the atom. Which of the following statements is true about the Bohr model of the atom.
N is a measure of the difference in energy between two energy levels. When energy is absorbed by atoms the electrons are promoted to higher-energy orbits. Learn vocabulary terms and more with flashcards games and other study tools.
The probability of finding an electron at a point within an atom is proportional to the ψ 2 at that point where ψ represents the wave-function of that electron. The electrons move around the protons which are at the center of the atom. N can have any positive whole-number value greater than or equal to 1.
According to Bohrs model of the atom orbits closer to the nucleus would require the electrons to have a greater amount of energy and orbits farther from the nucleus would require the electrons to have a smaller amount of energy.

Sound Really Is At The Foundation Of The Structure Of Matter Itself Learn More Http So Meditation Methods Personal Development Blog Posts Quantum Mechanics

No comments for "Which Statement Best Describes the Quantum Model of the Atom"
Post a Comment